An/Ln PARTITIONING AGENTS
Ligating extractants are effective in the separation of lanthanides from minor actinides. This step is essential for the partitioning and transmutation (P and T) component of the nuclear fuel cycle.
Item# FS-13
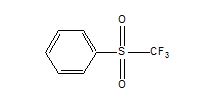
Compound Description: Phenyl Trifluoromethyl Sulfone
CAS# [426-58-4]
Phenyl trifluoromethyl sulfone (FS-13) is used as diluent or process solvent in nuclear applications, such as the UNEX process a, the HCCD-PEG process b, and the separation of minor actinides from lanthanides c .
(a) E.K.Rzhekhina, et al. (2007) “Processing of spent solvent of the UNEX process”. Radiochemistry, 49(5): 493-498.
(b) R.S. Herbst et al. (2008) “Fundamental chemistry of cesium extraction from acidic media by HCCD in FS-13” Solvent Extraction and Ion Exchange, 26(2):163-174.
(c) D.R. Peterman, et al. (2008) “Separation of minor actinides from lanthanides by dithiophosphinic acid extractants”. Solvent Extraction: Fundamentals to Industrial Applications: Proceedings of ISEC 2008 International Solvent Extraction Conference, Vol. 2, 1427-1432.
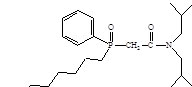
Item # CMPO
Compound Description: Octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide
CAS# [83242-95-9]
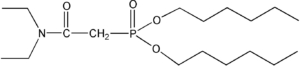
Item# DHDECMP
Compound Description: Dihexyl-N,N-diethylcarbamoylmethylenediphosphonate a.k.a N,N-diethylcarbamoylmethylphosphonic acid dihexyl ester
CAS# [7369-66-6]
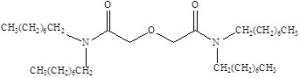
Item# TODGA
Compound Description: N,N,N’,N’-Tetraoctyl diglycolamide
CAS# [342794-43-8]
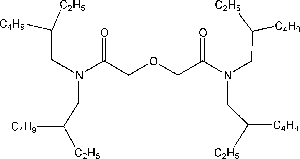
Item# DGA6
Compound Description: N,N’- bis(3,6-dimethyloctyl)-N’,N-bis(n-hexyl) diglycolamide
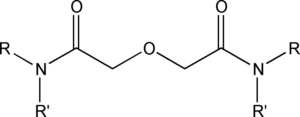
R= 3,6-dimethyloctyl, R’= n-hexyl
Item# T2EHDGA
Compound Description: N,N,N’,N’-Tetra(2-ethylhexyl) diglycolamide
CAS# [669087-46-1]
Item# DMDODGA
Compound Description: NN’-Dimethyl-N,N’-dioctyl diglycolamide
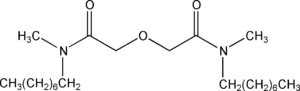
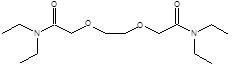
Item# DOODA(C1)
Compound Description: N, N,N’,N’-Tetramethyl-3,6-dioxaoctanediamide
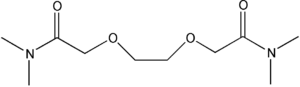
Item# DOODA(C2)
Compound Description: N, N,N’,N’-Tetraethyl-3,6-dioxaoctanediamide
CAS# [73796-39-1]
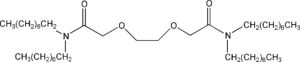
Item# DOODA(C8)
Compound Description: N, N,N’,N’-Tetraoctyl-3,6-dioxaoctanediamide
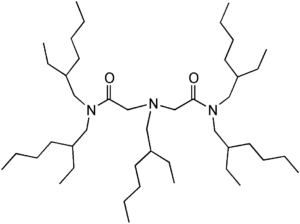
Item# ADAAM(EH)
Compound Description: 2-Ethylhexyl-Bis-(N’,N’-di(2-ethylhexyl)acetamido) amine
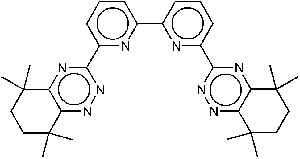
Item# CyMe4-BTBP
Compound Description: 6,6’-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3-yl)-2,2’-bipyridine
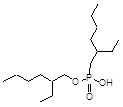
Item# HEH[EHP]
Compound Description: 2-Ethylhexylphosponic acid -2-ethylhexyl ester
CAS# [14802-03-0]
Item# DBBP
Compound Description:Di-n-butyl butylphosphonate
CAS# [78-46-6]
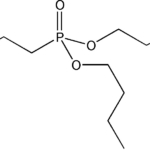
Item# DAAP
Compound Description: Di-n-amyl amylphosphonate
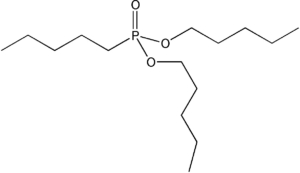
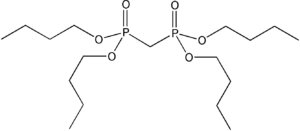
Item# TBMDP
Compound Description:Tetrabutylmethylenediphosphonate
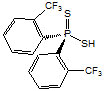
Item #: 50175
Compound Description: Bis(o-Trifluoromethylphenyl)dithiophosphinic acid
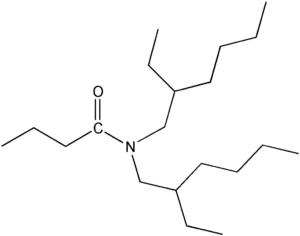
Item# DEHBA
Compound Description: N,N-Bis(2-ethylhexyl)butanamide
CAS# [112724-94-4]
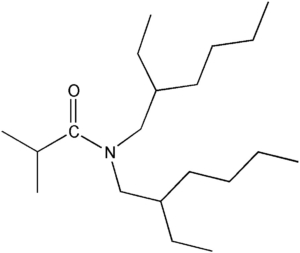
Item# DEHiBA
Compound Description:N,N-Bis(2-ethylhexyl)isobutanamide
CAS# [112724-95-5]
CMPO(a), DHDECMP(b), TODGA(c,d,e), T2EHDGA(e,f), DOODA(C8)(g), HEH[EHP](h), DAAP(i), TBMDP(j),CyMe4-BTBP(k) ADAAM(EH)(l), Bis(o-Trifluoromethylphenyl)-dithiophosphinic acid(m) and DEHBA(n,o) are examples of different classes of extractants with applications in the extraction and separation of lanthanides and actinides.
(a) R.C. Gatrone, et al. (1987) “The synthesis and purification of the carbamoylmethylphosphine oxides” Solvent Extraction and Ion Exchange, 5:1075-1116.
(b) J.N.Mathur et.al. (2001) “Actinide portioning –a review” Solvent Extraction and Ion Exchange 19(3): 357-390
(c) S.A. Ansari, et.al. (2005) “N,N,N’,N’-tetraoctyl diglycolamide (TODGA) : a promising extractant for actinide-partitioning from high-level waste (HLW)” Solvent Extraction and Ion Exchange 23(4): 463-479
(d) X. Sun; et.al. (2012) “Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle” Chem. Rev. 112(4):2100-2128
(e) A.V. Gelis, et.al. (2014) “Actinide lanthanide separation process-ALSEP” Industrial & Engineering Chemistry Research, 53: 1624-1631
(f) R.B.Gujar, et.al. (2010) “Development of T2EHDGA based process for actinide partitioning. Part I: Batch studies for process optimization” Solvent Extraction and Ion Exchange 28(3): 350-366
(g) Y.Sasaki, et.al. (2011) “Separation of Am, Cm, and lanthanides by solvent extraction with hydrophilic and lipophilic organic ligands” Solvent Extraction Research and Development, Japan, Vol. 18:93-101
(h) G.J.Lumetta (2010) “Review: Solvent systems combining neutral & acidic extractants for separating trivalent lanthanides from the transuranic elements” Solvent Extraction and Ion Exchange 28(3): 287-312
(i). B.J.Mincher (2014) “Characterizing diamylamylphosphonate (DAAP) as an Americium ligand for nuclear fuel-cycle applications” ” Solvent Extraction and Ion Exchange 32(2): 153-166
(j). A.Elias et.al. (1996) “Tetrabutylalkylenediphosphonates and tetrabutylalkylenediphosphonate-di(2-ethyl)phosponic acid mixtures in solvent extraction of uranyl nitrate”. Hydrometallurgya 40(1-2):189-194.
(k) A. Geist, et al. (2006) “6,6’-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3yl)-2,2’-bipyridine: an effective extracting agent for the separation of Americium(III) and curium(III) from the lanthanides” Solvent Extraction and Ion Exchange 24(4): 463-483
(l) H. Suzuki, et al. (2016) “Highly practical and simple ligand for separation of Am(III) and Eu(III) from highly acidic media” Analytical Sciences 32: 477-479
(m) J.R.Klaehan, et al. (2008) “Synthesis of symmetric dithiophosphinic acids for “minor actinide” extraction”. Inorganica Chimica Acta 361: 2522-2532.
(n). K.McCann et.al. (2017) “Hexavalent Actinide extraction using N,N-dialkylamides”. Industrial Engineering Chemistry Research 56(22): 6515-6519
(o) Hafeez, M. et al. J. Chem. Soc. Pak, 40(02), 2018